Brand
UScarePharm is dedicated to developing materials and products across diverse fields—including anti-aging technologies, aesthetic medicine, skin tissue regeneration, inner beauty, obesity, and hair loss—with the goal of becoming a global leader through technological innovation and exceptional quality.
View More
Platform Technology
Our dermatological science specialists participate directly in every stage, from functional ingredient development to final product formulation, to deliver safe and highly effective solutions. We focus on delivering innovative bio-health solutions that enhance quality of life while meeting the dual goals of beauty and health.
View More
ERBELLA
A name combining “Er” from Hermes, the messenger god in Greek mythology, and “Bella,” meaning beauty in Latin. ERBELLA is designed to deliver highly functional ingredients deep into the skin, to bring customers the ultimate beauty experience.
View More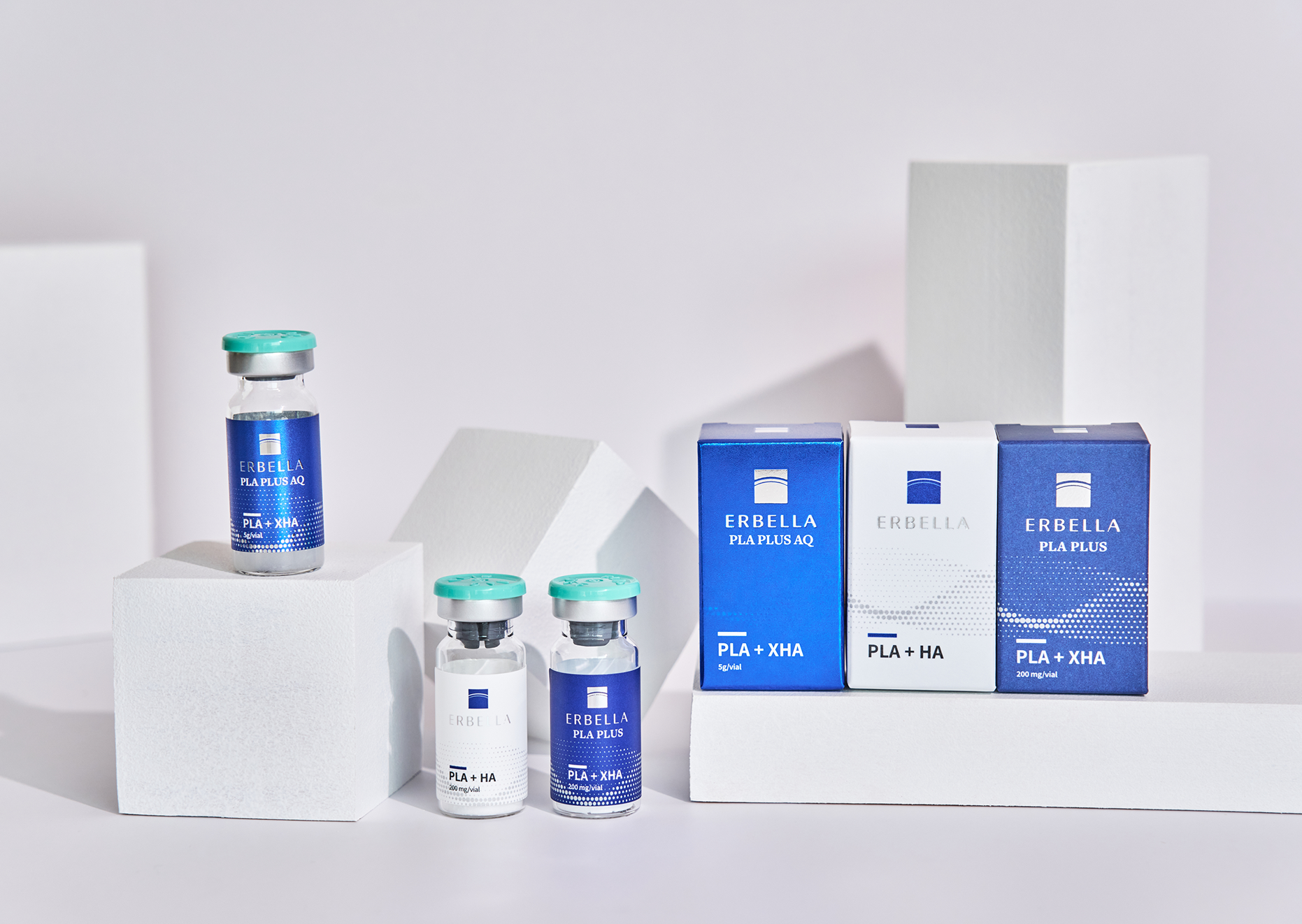
LLEAFILL
Developed through direct involvement of UScarePharm’s dermatological science specialists in every stage from functional ingredient research to product formulation, ensuring both safety and superior efficacy.
View More
Press Releases
-
‘Suwon K-DevHun Trade Mission’ Explores Export Opportunities in Czech Republic and the Netherlands
A scene of the “Suwon K-DevHun Trade Mission” engaging in export consultations with European buyers. / Photo courtesy of Suwon City Suwon Special City, together with local small and medium-sized enterprises (SMEs), has launched an export initiative in Europe—leveraging the global recognition of K-pop Demon Hunters (K-DevHun)—to pioneer new markets in the Czech Republic and the Netherlands. The city announced on the 13th that five local beauty and food manufacturing SMEs have formed the “Suwon K-DevHun Trade Mission,” which began export marketing activities with local buyers in the Czech Republic and the Netherlands on the 11th. The strategy taps into the worldwide popularity and strong fandom of the film K-pop Demon Hunters. The Netherlands and the Czech Republic, the destinations of the mission, rank No.1 in import volume per capita in Western and Eastern Europe respectively, making them key strategic hubs for EU trade expansion. Ahead of the trip, Suwon City identified a total of 50 prospective buyers across the two countries. The trade mission plans to visit each buyer’s business site to conduct export consultations and promote participating products—an average of 10 buyer visits per company. After meeting with Czech buyers on the 11th, the mission will continue visits with Dutch buyers from the 13th to the 14th. Participating companies include: UScarePharm (skin regeneration cosmetics) Cospaniel (salmon extract cosmetics)/SMEcolab (hand and foot nail care cosmetics)/Sungkyun Biotech (anti-fatigue health supplements)/HYERUM (tteokbokki meal kits) A Suwon City official stated, “We organized a specialized K-beauty and K-food export mission to utilize the explosive global popularity of K-DevHun as a growth engine in the EU market,” adding, “We will systematically support the expansion of K-beauty and K-food across major EU educational and commercial hubs such as the Netherlands and the Czech Republic.”
2025.11.17 09:41
-
Suwon Urban Foundation Dispatches “K-Beauty & K-Food Export Mission” to the Czech Republic and the Netherlands
▲ Members of the “2025 K-Beauty & Food Export Mission.” (Photo provided by the Suwon Urban Foundation) The Suwon Urban Foundation, in collaboration with Suwon City, has launched a strategic initiative to support local SMEs in expanding their presence in the European market. On the 12th, the foundation announced that the “2025 K-Beauty & Food Export Mission” has been dispatched to the Czech Republic and the Netherlands, with activities scheduled through the 16th. Five Suwon-based SMEs—Sungkyun Biotech, HYEYUM, SM Ecolab, UScarePharm, and Cospaniel—all recognized for their technological capabilities and market potential, are participating in the mission. The companies conducted business meetings with buyers in Prague, Czech Republic, and will continue one-on-one export consultations and on-site buyer visits in Amsterdam, the Netherlands, on the 13th and 14th. The consultation portfolio includes a range of consumer products in the K-Beauty and K-Food categories, such as health supplements, HMR (Home Meal Replacement) foods, functional wash products, and functional cosmetics. To support the mission, the Suwon Urban Foundation and Suwon City analyzed data from 50 local buyers in advance and established a comprehensive support system—including tailored buyer matching, interpretation services, and on-site meetups—to help participating companies effectively enter the European market. Lee Byung-jin, Director of the Suwon Urban Foundation, stated, “ This export initiative provides a meaningful platform for Suwon-based companies to present their strong product competitiveness directly to the European market. We will continue to strengthen our support to expand the global reach of the K-Beauty and K-Food industries.”
2025.11.17 09:39
-
ERBELLA Showcases Liquid PLLA and Cross-linked HA Innovation on the Middle Eastern Aesthetic Stage
Targeting the Middle East K-Medical Market with Natural Volume and Personalized Skin Boosters The medical aesthetics brand ERBELLA participated in Beautyworld Middle East 2025, held from October 27–29 at the Dubai World Trade Centre, presenting its signature product ERBELLA AQ along with an eight-product skin booster lineup that captured the attention of professionals across the Middle Eastern beauty and medical aesthetics industry. Beautyworld Middle East is the largest beauty and cosmetics exhibition in the region, featuring over 60 countries and hundreds of global brands each year. The 2025 edition highlighted cutting-edge innovations in K-beauty, skincare, medical aesthetics, and medical devices, with Korean brands earning strong recognition for their advanced technologies and product competitiveness in the Middle Eastern market. At its booth, ERBELLA introduced its innovative liquid-type PLLA filler “ERBELLA AQ” to major buyers and medical professionals from Dubai, Saudi Arabia, Qatar, and other regions, emphasizing its combination of natural volume and exceptional safety as key differentiators. ERBELLA AQ features a ready-to-use liquid formulation that combines Poly-L-lactic acid (PLLA) with cross-linked hyaluronic acid (HA), eliminating the need for a separate reconstitution process. This user-friendly formula enhances treatment convenience for practitioners while delivering instant hydration and volume upon injection. The cross-linked HA provides immediate plumping and moisture, while PLLA gradually stimulates collagen synthesis for long-lasting and natural volume improvement over time. Its stable liquid format minimizes the risk of nodules or granuloma formation, offering a high level of safety that meets the strict quality and stability standards of the Middle Eastern aesthetic market — a key reason why the product drew strong interest from local buyers. Beyond the AQ filler, ERBELLA also unveiled an eight-type skin booster lineup targeting specific skin needs such as firmness, antioxidant protection, and regeneration. Each formulation is designed for customized combination treatments depending on skin condition and procedure goals, positioning ERBELLA as a premium aesthetic brand suited for the step-by-step skincare programs favored in the Middle East. An ERBELLA representative commented, “ERBELLA AQ is more than a collagen stimulator — it’s a liquid PLLA solution that delivers natural, long-lasting volume. Our skin booster lineup further represents our identity as a total aesthetic care brand focused on comprehensive skin health and beauty.” “Through this exhibition, we aim to redefine the standards of K-Medical technology and beauty across the Middle East and the global market.” The Middle East has recently emerged as one of the fastest-growing regions in the aesthetic and cosmetic medical industry, with soaring demand for K-beauty and K-medical products. Building on successful partnerships and distribution agreements in Saudi Arabia and Iraq, ERBELLA is accelerating its expansion throughout the region. Following its participation in Beautyworld Middle East, the brand plans to further strengthen local partnerships and distribution networks to solidify its market presence. Source: E-Donga (https://edu.donga.com)
2025.11.11 15:39
Inquiry
UScarePharm continues to innovate every day for healthy beauty.






